This post covers a brief history of the Universe from the Big Bang until the the present day. This, as I am sure you’ll agree, is a pretty big topic so I’ll only give a outline of some of the key events and when we believed they happened. This post in my series about cosmology, which is the study of the origin and evolution of the Universe as a whole. To view the others click on https://explainingscience.org/tag/cosmology/
Before I start talking about the history of the Universe I first need to give a brief overview of atoms, which are the building blocks of all matter which we see in our everyday lives
Background – atoms
An atom consists of a nucleus, which has a positive electric charge, surrounded by negatively charged electrons. Atoms are very small, typically around 0.0001 microns in diameter (a micron is a millionth of a metre). However the nucleus, which contains nearly all the mass of the atom, is much, much smaller, typically around 0.000 000 001 microns in diameter. This means that the nucleus is one hundredth thousandth of the diameter of the whole atom.
The nucleus consists of a number of positively charged protons and neutrons which have no charge. Because the electrons have a negative charge, and the number of protons and electrons in an atom is always the same, the atom has a net charge of zero.
- The number of protons is called the atomic number and determines which element it is. You may remember from high school chemistry that this is its position in the periodic table.
- The number of neutrons in the nucleus does not affect the chemical properties of the atoms. In fact, all elements have different versions of themselves called isotopes, which have a different numbers of neutrons but the same number of protons.
The simplest possible atomic nucleus is that of hydrogen, which consists of a single proton. Atoms which have 2 protons (regardless of the number of neutrons) are helium atoms, 3 protons are lithium atoms and so on. The element with the highest atomic number, which naturally occurs on Earth, is uranium, which has 92 protons.
A atom of the most common isotope of carbon has 6 protons and 6 neutrons in the nucleus surrounded by 6 electrons.
The first second after the Big Bang
There is no generally agreed account of what happened at the exact instant of the Big Bang. If we work backward in time, using our existing physical theories, then at the exact instant of creation the Universe would have had an infinite density and an infinite temperature. What this really means is that we don’t have a physical theory which explains what happened at this time.
What is generally accepted in that in the first microscopic fraction of a second, after the Universe first came into existence, it underwent an incredibly rapid expansion and has been expanding and cooling ever since (see notes 1 below). For the first part of the first second, the conditions in the Universe were so hot and dense that it consisted only of some special particles. These cannot be detected under normal conditions, but you may have heard about the recently discovered Higgs Boson, which can be very briefly seen in the extreme conditions created in particle accelerators, like the Large Hadron Collider in Switzerland. The Higgs Boson is just one example of the particles which would have been present during the first minute fraction of a second.
When it was one second old the Universe was expanding and cooling rapidly. However it was still at a temperature of around 1 trillion degrees Centigrade. At this point the ordinary matter in the Universe consisted of a sea of protons, neutrons and electrons. None of these particles were bound together into atoms, because atoms cannot exist at such high temperatures (see notes 2 below).
10 to 1000 seconds after the Big Bang
At around 10 seconds after the Big Bang the Universe had cooled to 1 billion degrees. This is cool enough for atomic nuclei to exist. In fact, the whole Universe acted as a giant nuclear reactor. Hydrogen nuclei were turned in helium nuclei in a three stage process. There are two main routes in the reaction, but the end result is the same. Two protons (hydrogen nuclei) and two neutrons are fused into a helium nucleus releasing a vast amount of energy
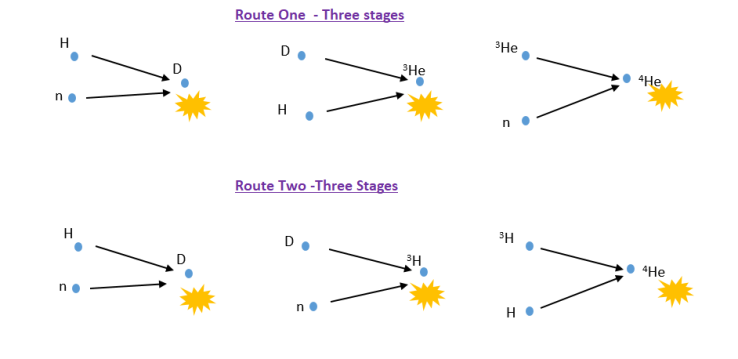
Even though the reactions generated a huge amount of energy, the cooling produced by the expansion of the Universe was so rapid that when the Universe was around 1000 seconds old it had cooled to around 10 million degrees and was no longer hot enough and dense enough for any further nuclear reactions to take place. At this time the matter in the Universe consisted of 73% hydrogen and 27% helium with trace amounts of deuterium, lithium and beryllium. None of the heavier elements existed. There were all created later by nuclear reactions inside stars.
Atoms form
The early Universe was so hot that the matter was in a special state which we call a plasma. In a plasma in the electrons are not bound to the atomic nucleus to form atoms but can move around freely. Light cannot pass through the plasma, which would have been like a hot dense glowing fog.
A plasma
However, as the Universe continued to expand and cool it reached a temperature where helium atoms could exist. Later, when it was roughly 400, 000 years old, and at a temperature of around 3,000 degrees, ordinary hydrogen atoms could exist and the Universe became transparent to light. The faint radiation which we can observe today called the cosmic microwave background was created at this time.
First Stars Form
As the Universe continued to expand and cool, matter began to clump together. When it was about 100-150 million years old, about 1% of its current age, large clumps of matter existed which were around 100 to 300 times the mass of of Sun. These clumps of matter contracted, getting hotter and hotter as they did so. Eventually they were so hot that nuclear reactions could start, and thus the first stars were born. These early stars, which astronomers call population III stars, were super massive compared to the Sun and shone extremely brightly for about 10 million years. (This is a very short lifetime for a star, as the Sun will last for about 10 billion years.) They ended their lives in massive explosions called supernovae in which the star was completely destroyed. These supernovae spread the elements made in the star – like carbon, nitrogen, oxygen, silicon, magnesium, iron and uranium – throughout the Universe.
Galaxies Form
It is still not fully understood how galaxies , which contain hundreds of billions of stars, form. One theory which has gained strength in recent years is sometimes called the “bottom up” theory. According to this theory the first galaxies began to form when the Universe was around 1 billion years old from lumps of matter, including stars and gas clouds which had coalesced.
As our universe continues to evolve, small galaxies are frequently gobbled up by larger ones. The Milky Way contains the remains of several smaller galaxies that it has swallowed during its long lifetime. In fact, the Milky Way is “digesting” at least two small galaxies even now, and may pull in others over the next few billion years.
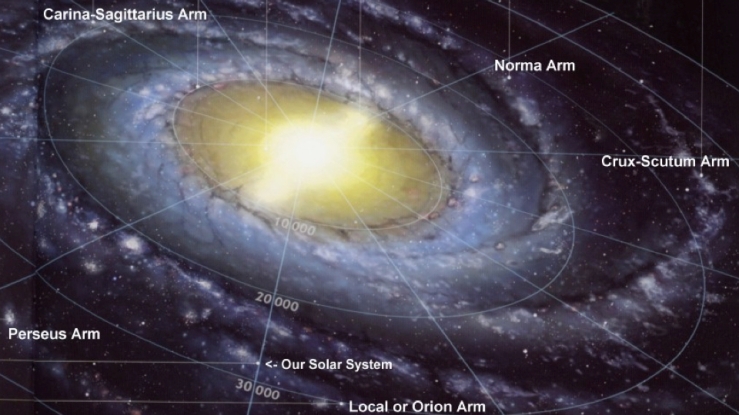
Milky way structure Population II and Population I Stars
Our own Milky Way galaxy, shown below, is believed to date from just over 1 billion years from the creation of the Universe.
Around the Milky Way is a halo containing old stars, called Population ll stars, which do not have many elements other than hydrogen and helium. The traces of the heavier elements will have come from the earlier Population III stars which exploded, as discussed above. When stars are formed, the materials left over combine to form planets. Because elements such as iron, oxygen and silicon are only present in tiny quantities in these Population II stars, they cannot be orbited by rocky planets like the Earth, Mars and Venus. The disk of the galaxy and the central bulge contain younger population I stars, such the Sun. These are much richer in heavier elements and are likely to have planets.
The Ultimate in Recycling ?
Much of the material in the Population I stars and their planets has been re-cycled. It will have been created in earlier Population II (and Population III) stars which exploded as supernovae, scattering the debris throughout the Universe. As mentioned above, some of the debris later clumps together to form stars and planets, such as the Earth.

And finally….
I hope you’ve enjoyed this post. If you want to find out more about Explaining Science, please check out my YouTube Channel https://www.youtube.com/explainingscience
Updated 28 June 2021
Notes
1 This is called inflation and is accepted by most cosmologists. In this theory the part of the Universe we inhabit expanded from a minute fraction of the size of an atomic nucleus to a diameter of about 1 metre in a period of around 0.00000000000000000000000000000001 seconds after the instant of creation.
2. Most cosmologists now believe that 85% of the matter in the Universe is another mysterious form of matter called dark matter. No one knows what dark matter consists of, but is clear is that it not made up of atoms in the same way as ordinary matter. Dark matter cannot form stars and does not clump together to form structures like gas clouds in the same way that ordinary matter does. It is completely invisible to telescopes because it is transparent to light. However its existence is inferred because of its gravitational effects on visible matter. Dark matter is covered in my post https://explainingscience.org/2015/02/18/dark-matter/

[…] to the standard big bang theory ,the Universe is 13.77 billion years old and began in a phase of extremely high (possibly infinite) […]
LikeLike
[…] reality, we cannot see all the way to the particle horizon. As readers of my earlier post will know, the early Universe was far too hot for atoms to exist. It contained a plasma of […]
LikeLike
[…] readers of my earlier post will know, the early Universe was far too hot for atoms to exist. It contained a plasma of […]
LikeLike
Reblogged this on ItsNotWhatItsWhy.
LikeLike
[…] readers of my previous post will know, the generally accepted theory of the origin of the Universe is that it was created in an […]
LikeLike
This is an awesome post! I thoroughly enjoyed reading it!
LikeLike
[…] Before I talk about how the Sun generates its energy I’ll give a brief overview of atoms. For more details see my previous post: A Brief History of the Universe. […]
LikeLike
[…] A Brief History of the Universe. […]
LikeLike
[…] you will recall from my previous post ordinary matter is made up of atoms. Some dark matter may be in the form of ordinary matter in […]
LikeLike
Next time I need to explain it, vis-a-vis one of my plots, I’ll direct my readers here. I never could condense it. I get stuck on the Big Bang and all that repulsive gravity.
LikeLike
Thanks for your comment. I am planning at least four more posts on cosmology. So hopefully they’ll prove just as usefull.
The Science Geek
LikeLiked by 1 person
[…] (2) A brief history of the Universe. This gives a history of the Universe from just after the big bang until the current date. To view this post click here. […]
LikeLike
[…] (2) A brief history of the Universe.This gives a history of the Universe from just after the big bang until the current date To view this post click here. […]
LikeLike
Reblogged this on athisayam29 and commented:
Loved the way this briefing lined for enthusiasts to get basic idea
LikeLike
Wow! You explain in a very good way! Congratulations!
LikeLike
Our whole universe was in a hot dense space. Lol
All I could think of was the theme song for the Big Bang Theory
LikeLike
Reblogged this on bertpowers and commented:
Good information.
LikeLike
Great one! As some one with a working but still largely incomplete idea of the history of the universe, this was probably written just for me. Also thanks for following at Tower of buckets and you should probably check out http://towerofbuckets.wordpress.com/2014/11/24/breaking-the-speed-limit/ . I get the impression you’d like it, even if the physics is dubious bordering on completely made up. There’s no way of putting that nicely. Hope you keep posting!
LikeLike
Thanks for your comment and glad you found my post interesting. I’ll take a look at your link.
As I’ve mentioned in previous replies, I found it difficult to write a post about the entire history of the Universe from the big bang onwards and get the balance right between giving too much information, and putting off non scientists, but still managing to get the key facts across.
.
The Science Geek
LikeLike
Reblogged this on SandraBranum's Blog and commented:
I love The Big Bang Theory and whether you agree it not, it IS interesting.
LikeLike
Reblogged this on James' World 2.
LikeLike
Hi there Science Geek, really like the site. Content is well chosen and the site has a nice clean look. Looking forward to future posts and will be working my way through the older ones. Keep us informed 😉
LikeLike
The physics of the early universe is fascinating. A paradox of Big Bang theory is that it should have produced equal amounts of matter and antimatter, which naturally would have completely annihilated each other. This is called the baryon asymmetry problem. I don’t know too much about this, but here’s a Wikipedia link to a partial explanation: http://en.wikipedia.org/wiki/Leptogenesis_(physics). It’s really interesting!
LikeLike
Thank you for the link. Very interesting !
LikeLike
Very good. Not so theoretical and geeky that the average layman can’t understand but detailed enough that scientists will probably say it’s a pretty good summation.
LikeLike
Thanks for your comment,
I have found it difficult to write a post about the entire history of the Universe and get the balance right between overloading readers with too much information, and putting off non scientists, but still managaging to get the essential information across.
Hopefully I’ve hit the spot.
The Science Geek
LikeLike
Thanks. I am considering those as future topics for posts.
The Science Geek
LikeLike
That was a seriously good post. How about an article on the fundamental forces and the theory of everything? Perhaps in bite sized chunks.
LikeLike